Abstract
Background: Myelodysplastic syndromes (MDS) are a group of clonal hematopoietic stem cell malignancies defined by ineffective hematopoiesis and peripheral blood cytopenias. Patients often present with anemia and frequently require red blood cell transfusions (RBCTs), which may lead them to become transfusion-dependent (TD). This results in poor quality of life and reduced survival. In patients with non-del(5q) lower-risk (LR)-MDS, the standard of care includes erythropoiesis-stimulating agents (ESAs) ± granulocyte colony-stimulating factor as first-line (1L) therapy; however, although clinical trials have demonstrated efficacy, there is little evidence from real-world settings. This study aimed to assess real-world data to investigate treatment patterns and prevailing hematologic outcomes in patients with non-del(5q) LR-MDS.
Methods: In this retrospective medical record review, eligible patients ≥ 18 years of age diagnosed with non-del(5q) LR-MDS between July 1, 2013 and September 30, 2018 were identified by participating hematologist-oncologists in the US, Canada (CAN), UK, and EU (France, Germany, Spain). Demographics, clinical characteristics, treatment patterns, and hematologic outcomes data were abstracted (June-November 2021) and reported descriptively. Aggregated analyses were performed using pooled US/CAN and UK/EU data sets for the overall cohort and for patient subgroups treated with clinician-defined 1L and second-line (2L) ESA-containing regimens.
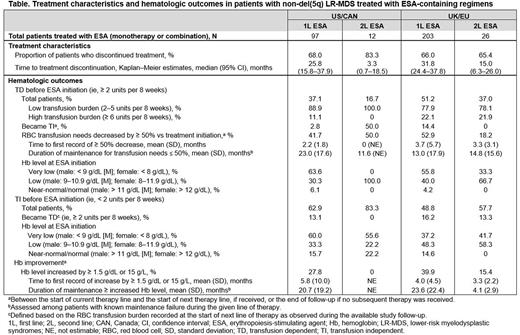
Results: Medical record data were abstracted for 119 patients with non-del(5q) LR-MDS in US/CAN (median age, 61.5 years; 71.4% male) and 245 patients in UK/EU (median age, 67.3 years; 71.4% male). Median follow-up duration from MDS diagnosis was 50.5 months (US/CAN) and 67.8 months (UK/EU). At initial diagnosis, 50.4% (US/CAN) and 53.5% (UK/EU) of patients had Low-risk MDS (per Revised International Prognostic Scoring System). Of the 71 patients in US/CAN and 123 patients in UK/EU who were tested successfully for the SF3B1 mutation, 16.9% (US/CAN) and 15.5% (UK/EU) were positive. Most of the patients initiated 1L therapy (US/CAN: 109 [91.6%]; UK/EU: 225 [91.8%]) and more than one-third initiated a 2L therapy (US/CAN: 45 [37.8%]; UK/EU: 89 [36.3%]). In 1L, 2.8% patients in US/CAN and 2.2% in UK/EU received treatment with lenalidomide. Most patients were treated with ESA-containing regimens in 1L, with a median duration of therapy of ≥ 2 years (US/CAN: 97 [89.0%], of which 86.6% was ESA monotherapy; UK/EU: 203 [90.2%], of which 85.7% was ESA monotherapy). Treatment characteristics and hematologic outcomes for patients treated with ESAs are presented in the Table. Among patients who were TD before 1L ESA treatment (36 [37.1%] US/CAN; 104 [51.2%] UK/EU), a small proportion (2.8% US/CAN; 14.4% UK/EU) became transfusion independent (TI) during 1L; RBCT reduction threshold (≥ 50%) was met by 41.7% in US/CAN (median duration, 23 months) and 52.9% in UK/EU (median duration, 13 months). Among patients treated with ESA-containing regimens in 2L (12 [26.7%] US/CAN; 26 [29.2%] UK/EU), most had been treated with ESA in 1L (US/CAN: 10 [83.3%]; UK/EU: 22 [84.6%]). The most frequently reported reasons for reinitiating treatment with ESAs in 2L were overall health status (54.6% US/CAN; 40.9% UK/EU), treatment efficacy (45.5% US/CAN; 63.6% UK/EU), and disease characteristics (36.4% US/CAN; 45.5% UK/EU). In the subgroup of patients who reinitiated ESA in 2L, the most frequently reported reasons for discontinuation of 1L ESA were patient decision (45.5% US/CAN; 22.7% UK/EU), completion of planned course of therapy (45.5% US/CAN; 18.2% UK/EU), and progressive disease (36.4% US/CAN; 68.2% UK/EU).
Conclusions: In this real-world study, the most frequently used treatments for the management of anemia in patients with non-del(5q) LR-MDS were ESA-containing regimens. A considerable proportion of patients were TD before initiating 1L ESA therapy. For those who were re-treated with ESAs in 2L, the most common reasons for treatment reinitiation were health status, disease characteristics, and reported treatment efficacy, although only a small fraction of patients who were TD at 1L ESA initiation became TI. These findings highlight an unmet need in anemia management among patients with non-del(5q) LR-MDS, and a requirement for new and more effective treatment options.
Disclosures
Diez-Campelo:Bristol Myers Squibb: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees, Research Funding; Novartis: Consultancy, Honoraria, Membership on an entity's Board of Directors or advisory committees; Takeda: Honoraria, Membership on an entity's Board of Directors or advisory committees; BluePrint: Membership on an entity's Board of Directors or advisory committees. Yucel:Bristol Myers Squibb: Current Employment, Current holder of stock options in a privately-held company. Goyal:BMS: Other: Full time employee of RTI Health Solutions, which is a business unit of RTI International, a non-profit research organization. RTI Health Solutions received funding from BMS for the conduct of this study. Parikh:BMS: Other: Full time employee of RTI Health Solutions, which is a business unit of RTI International, a non-profit research organization. RTI Health Solutions received funding from BMS for the conduct of this study.. Esterberg:BMS: Other: Full time employee of RTI Health Solutions, which is a business unit of RTI International, a non-profit research organization. RTI Health Solutions received funding from BMS for the conduct of this study.. Jimenez:Bristol Myers Squibb: Research Funding. Miteva:BMS: Current Employment. Xiao:Bristol Myers Squibb: Current Employment, Current equity holder in private company. Germing:Celgene: Consultancy, Honoraria, Research Funding; Novartis: Consultancy, Honoraria.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal